All Photos(1)
About This Item
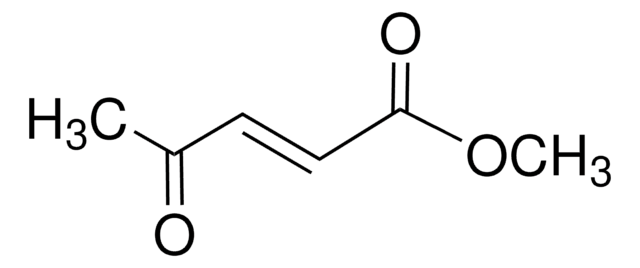
Linear Formula:
C2H5CH=C(CH3)CO2CH3
CAS Number:
Molecular Weight:
128.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
liquid
refractive index
n20/D 1.438 (lit.)
bp
46-47 °C/15 mmHg (lit.)
density
0.92 g/mL at 25 °C (lit.)
SMILES string
CC\C=C(/C)C(=O)OC
InChI
1S/C7H12O2/c1-4-5-6(2)7(8)9-3/h5H,4H2,1-3H3/b6-5+
InChI key
SRORRGMOEUTSDV-AATRIKPKSA-N
General description
Methyl trans-2-methyl-2-pentenoate is an α,β-unsaturated ester. It is formed during the photolysis of pyrazoline. Methyl cis- and trans-2-methyl-2-pentenoate are formed during the pyrolysis of cis- and trans-3,5-dimethyl-3-carbomethoxy-Δ1-pyrazoline.
Application
Methyl trans-2-methyl-2-pentenoate may be used in chemical synthesis.
signalword
Warning
hcodes
Hazard Classifications
Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
123.8 °F - closed cup
flash_point_c
51 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
PYRAZOLINES: PART III. THE PREPARATION AND PYROLYSIS OF 4, 5-DIMETHYL-3-CARBOMETHOXY-?2-PYRAZOLINE AND 3, 5-DIMETHYL-3-CARBOMETHOXY-?1-PYRAZOLINE.
McGreer DE, et al.
Canadian Journal of Chemistry, 41(3), 726-731 (1963)
Pyrazolines. VII. Concerning the formation of olefins from the pyrolysis of pyrazolines.
McGreer DE and Wu W-S.
Canadian Journal of Chemistry, 45(5), 461-468 (1967)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service