342157
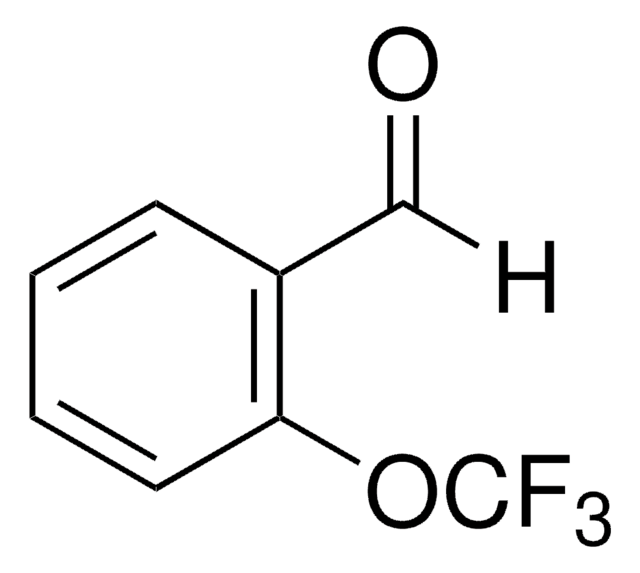
2-Hydroxy-5-(trifluoromethoxy)benzaldehyde
99%
Synonym(s):
5-(Trifluoromethoxy)salicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3OC6H3(OH)CHO
CAS Number:
Molecular Weight:
206.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
solid
assay:
99%
Recommended Products
assay
99%
form
solid
bp
82 °C/60 mmHg (lit.)
mp
31-33 °C (lit.)
SMILES string
Oc1ccc(OC(F)(F)F)cc1C=O
InChI
1S/C8H5F3O3/c9-8(10,11)14-6-1-2-7(13)5(3-6)4-12/h1-4,13H
InChI key
WQUZBERVMUEJTD-UHFFFAOYSA-N
General description
2-Hydroxy-5-(trifluoromethoxy)benzaldehyde is formed as intermediate during the biotransformation pathways of CP-122,721 in humans.
Application
2-Hydroxy-5-(trifluoromethoxy)benzaldehyde may be used in the preparation of :
- 2-[(E)-2-hydroxy-5-(trifluoromethoxy)benzylideneamino]-4-methylphenol
- (E)-2-((3-fluorophenylimino)methyl)-4-(trifluoromethoxy) phenol
- 2-[(E)-(naphthalen-2-ylimino) methyl]-4-(trifluoromethoxy) phenol
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aslı Tosyalı Karadağ et al.
Acta crystallographica. Section E, Structure reports online, 67(Pt 1), o95-o95 (2010-01-01)
The title compound, C(15)H(12)F(3)NO(3), is a Schiff base which adopts the cis-quinoid form in the solid state. The dihedral angle between the least-squares planes of the benzene rings being 3.6 (1)°. The F atoms of the -CF(3) group are disordered over
Yelda Bingöl Alpaslan et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 3), o510-o510 (2010-01-01)
The title compound, C(14)H(9)F(4)NO(2), is a Schiff base which adopts the phenol-imine tautomeric form in the solid state. The H atom is located on the hydr-oxy O atom rather than on the N atom. This H atom is involved in
Kevin Colizza et al.
Drug metabolism and disposition: the biological fate of chemicals, 35(6), 884-897 (2007-03-16)
The metabolism, pharmacokinetics, and excretion of a potent and selective substance P receptor antagonist, CP-122,721 [(+)-(2S,3S)-3-(2-methoxy-5-trifluoromethoxybenzylamino)-2-phenylpiperidine], have been studied in six healthy male human subjects [four extensive metabolizers (EMs) and two poor metabolizers (PMs) of CYP2D6) following oral administration of
2-[(E)-(Naphthalen-2-ylimino) methyl]-4-(trifluoromethoxy) phenol.
Pekdemir M, et al.
Acta Crystallographica Section E, Structure Reports Online, 68(4), o10204-o10204 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service