All Photos(1)
About This Item
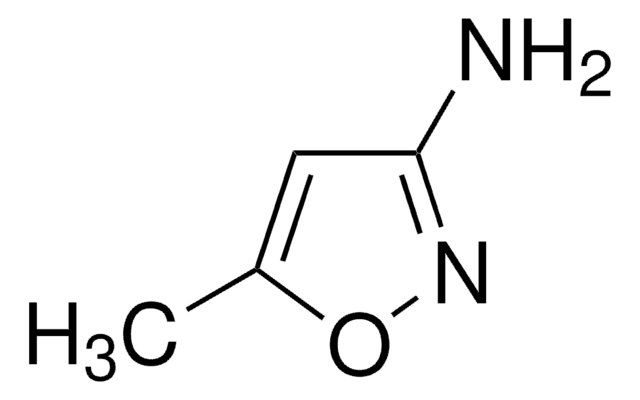
Empirical Formula (Hill Notation):
C5H6N2O3
CAS Number:
Molecular Weight:
142.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
67-69 °C (lit.)
functional group
nitro
SMILES string
Cc1noc(C)c1[N+]([O-])=O
InChI
1S/C5H6N2O3/c1-3-5(7(8)9)4(2)10-6-3/h1-2H3
InChI key
PMQFLWLYAXYFHG-UHFFFAOYSA-N
Application
3,5-Dimethyl-4-nitroisoxazole was used in the synthesis of:
- series of methylene bis-isoxazolo[4,5-b]azepines, via reaction with methylene bis-chalcones
- phenylmethylene bis-isoxazolo[4,5-b]azepine derivatives
- 3-arylglutaric acids, bis-isoxazoles and bis-pyrazoles
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Rajanarendar et al.
Bioorganic & medicinal chemistry letters, 22(1), 149-153 (2011-12-14)
A series of novel phenylmethylene bis-isoxazolo[4,5-b]azepine derivatives (10) have been synthesized from 3-methyl-4-nitro-5-styrylisoxazoles 6. The reaction of 6 with 3,5-dimethyl-4-nitroisoxazole (7) in piperidine afforded the Michael type adducts 8, which on treatment with different substituted chalcones in the presence of
Three multicomponent reactions of 3, 5-dimethyl-4-nitroisoxazole.
Adamo MFA, et al.
Tetrahedron, 63(39), 9741-9745 (2007)
E Rajanarendar et al.
European journal of medicinal chemistry, 50, 344-349 (2012-03-06)
A series of novel methylene bis-isoxazolo[4,5-b]azepines have been synthesized by reaction of 3,5-dimethyl-4-nitroisoxazole 6 with an appropriate methylene bis-chalcones 7 to obtain various Michael adducts 8a-i, which on treatment with SnCl(2)-MeOH underwent reductive cyclization to afford the title compounds 9a-i.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service