330191
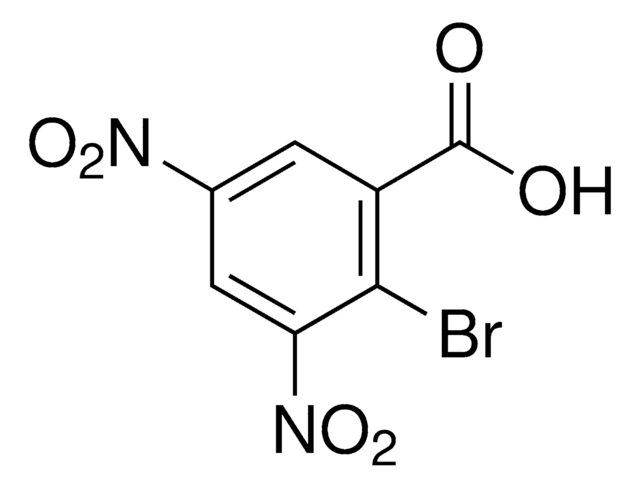
2-Bromo-3-nitrobenzoic acid
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
246.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
assay
90%
mp
184-186 °C (lit.)
functional group
bromo
carboxylic acid
nitro
SMILES string
OC(=O)c1cccc(c1Br)[N+]([O-])=O
InChI
1S/C7H4BrNO4/c8-6-4(7(10)11)2-1-3-5(6)9(12)13/h1-3H,(H,10,11)
InChI key
WTDJEGSXLFHZPY-UHFFFAOYSA-N
Application
2-Bromo-3-nitrobenzoic acid was used in preparation of 3-substituted 5-nitroisocoumarins and 2-bromo-3-nitrobenzaldehyde.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Esther C Y Woon et al.
Bioorganic & medicinal chemistry, 21(17), 5218-5227 (2013-07-16)
Poly(ADP-ribose)polymerase-1 (PARP-1) is an important target for drug design for several therapeutic applications. 5-Aminoisoquinolin-1-one (5-AIQ) is a highly water-soluble lead compound; synthetic routes to 3-substituted analogues were explored. Tandem Hurtley coupling of β-diketones with 2-bromo-3-nitrobenzoic acid, retro-Claisen acyl cleavage and
Preparation and NMR analysis of 2, 6-heterodifunctional halobenzenes as precursors for substituted biphenyls
Sienkowska M, et al.
Tetrahedron, 56(2), 165-173 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service