All Photos(1)
About This Item
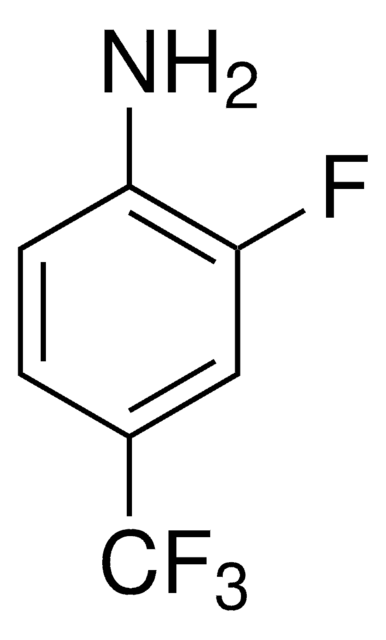
Linear Formula:
F3C6H2NH2
CAS Number:
Molecular Weight:
147.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
99%
mp
59-63 °C (lit.)
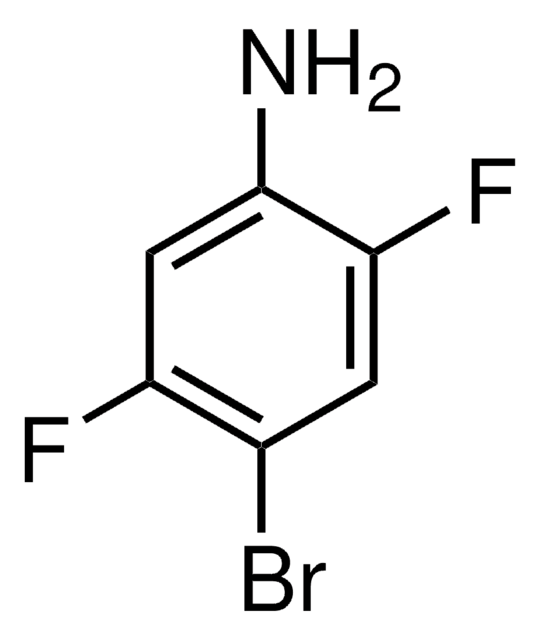
SMILES string
Nc1cc(F)c(F)cc1F
InChI
1S/C6H4F3N/c7-3-1-5(9)6(10)2-4(3)8/h1-2H,10H2
InChI key
QMYVWJVVVMIBMM-UHFFFAOYSA-N
Application
2,4,5-Trifluoroaniline was used in the synthesis of 1,2,4-trifluoro-5-nitrobenzene by reacting with acetonitrile and aqueous acetonitrile.
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Christopher B McPake et al.
ChemSusChem, 5(2), 312-319 (2011-12-16)
The generation and use of the highly potent oxidising agent HOF·MeCN in a controlled single continuous flow process is described. Oxidations of amines and azides to corresponding nitrated systems by using fluorine gas, water and acetonitrile by sequential gas-liquid/liquid-liquid continuous
Mohamad Shazwan Shah Jamil et al.
Dalton transactions (Cambridge, England : 2003), 48(25), 9317-9327 (2019-06-06)
A series of imidazolium salts precursors for N-heterocyclic carbenes (NHCs) featuring fluoroaryl substituents have been prepared along with their selenides and rhodium complexes. Tests of the catalytic activity of the [Rh(cod)Cl(NHC)] complexes in the transfer hydrogenation of acetophenone with iPrOH
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service