301140
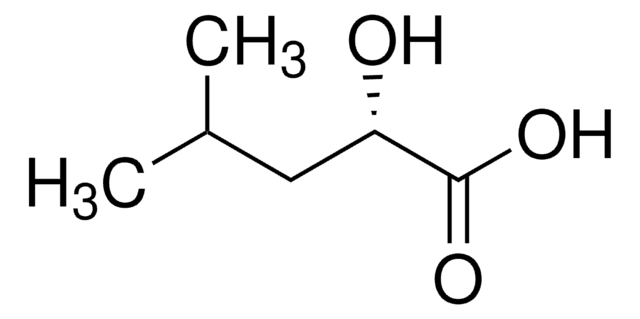
(R)-(−)-Hexahydromandelic acid
98%, optical purity ee: 99% (GLC)
Synonym(s):
(R)-(−)-Cyclohexylhydroxyacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H11CH(OH)CO2H
CAS Number:
Molecular Weight:
158.19
Beilstein/REAXYS Number:
3196809
MDL number:
UNSPSC Code:
51113400
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
optical activity
[α]18/D −23°, c = 1 in acetic acid
optical purity
ee: 99% (GLC)
mp
127-129 °C (lit.)
functional group
carboxylic acid
hydroxyl
SMILES string
O[C@H](C1CCCCC1)C(O)=O
InChI
1S/C8H14O3/c9-7(8(10)11)6-4-2-1-3-5-6/h6-7,9H,1-5H2,(H,10,11)/t7-/m1/s1
InChI key
RRDPWAPIJGSANI-SSDOTTSWSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(R)-(−)-Hexahydromandelic acid can be used:
- As an intermediate in the synthesis of antimycobacterial compound pyridomycin analogs.
- As a model α-hydroxy acid compound in the chirality sensing studies of organic probes using circular dichroism technique.
- As a starting material in the synthesis of chiral ionic liquids, which are applicable as chiral solvents in asymmetric synthesis and as chiral stationary phases in chromatographic separations.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chiral ionic liquids: synthesis, properties, and enantiomeric recognition
Yu S, et al.
The Journal of Organic Chemistry, 73(7), 2576-2591 (2008)
Synthesis and Structure-Activity Relationship Studies of C2-Modified Analogs of the Antimycobacterial Natural Product Pyridomycin
Kienle M, et al.
Journal of medicinal chemistry, 63(3), 1105-1131 (2020)
Chirality sensing of amines, diamines, amino acids, amino alcohols, and ?-hydroxy acids with a single probe
Bentley KW, et al.
Journal of the American Chemical Society, 135(48), 18052-18055 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service