291129
L-Ascorbic acid 6-palmitate
95%
Synonym(s):
6-O-Palmitoyl-L-ascorbic acid, L-Ascorbyl palmitate, Ascorbic acid 6-palmitate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H38O7
CAS Number:
Molecular Weight:
414.53
Beilstein/REAXYS Number:
96552
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
assay
95%
optical activity
[α]20/D +22.9°, c = 2 in methanol
mp
115-118 °C (lit.)
SMILES string
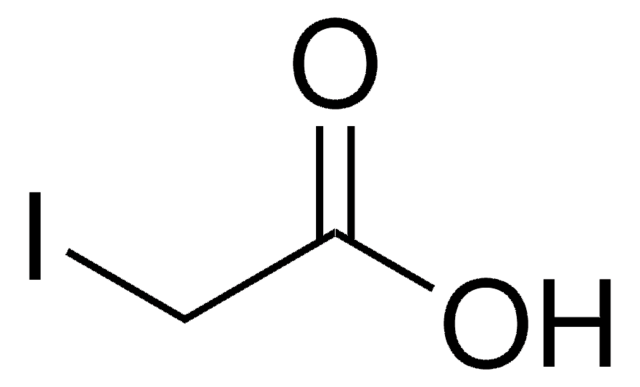
[H][C@@]1(OC(=O)C(O)=C1O)[C@@H](O)COC(=O)CCCCCCCCCCCCCCC
InChI
1S/C22H38O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(24)28-16-17(23)21-19(25)20(26)22(27)29-21/h17,21,23,25-26H,2-16H2,1H3/t17-,21+/m0/s1
InChI key
QAQJMLQRFWZOBN-LAUBAEHRSA-N
Looking for similar products? Visit Product Comparison Guide
replaced by
Product No.
Description
Pricing
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
O P Bamidele et al.
Carbohydrate polymers, 216, 217-223 (2019-05-03)
This study determines storage stability and release of encapsulated ascorbyl palmitate in normal and high amylose maize starch by pasting and spray drying. The amount of ascorbyl palmitate released was analysed in the stored samples (dark cupboard, and under UV
Martin Spickenreither et al.
Bioorganic & medicinal chemistry letters, 16(20), 5313-5316 (2006-08-16)
Previously, we identified ascorbic acid 6-O-hexadecanoate as an up to 1500 times more potent inhibitor of bacterial and bovine hyaluronidases than the parent compound, vitamin C, and determined a crystal structure of hyaluronidase from Streptococcus pneumoniae in complex with the
Stephan Braun et al.
European journal of medicinal chemistry, 46(9), 4419-4429 (2011-08-02)
Bacterial hyaluronan lyases (Hyal) degrade hyaluronan, an important component of the extracellular matrix, and are involved in microbial spread. Hyal inhibitors may serve as tools to study the role of the enzyme, its substrates and products in the course of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service