All Photos(1)
About This Item
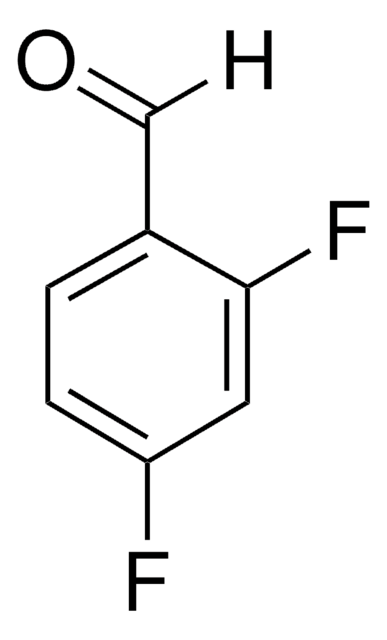
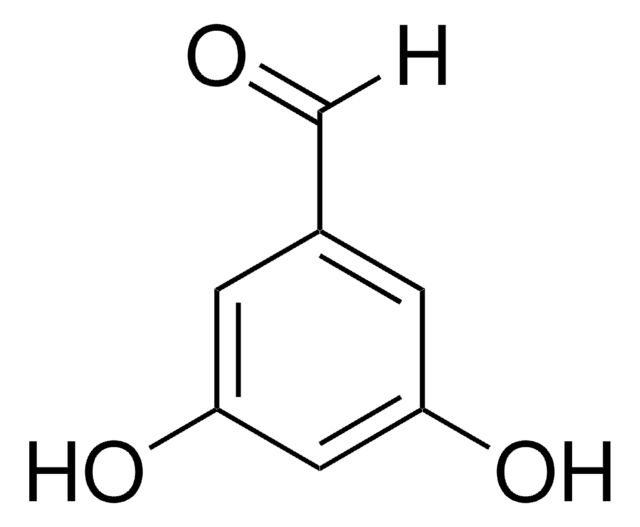
Linear Formula:
F2C6H3CHO
CAS Number:
Molecular Weight:
142.10
Beilstein/REAXYS Number:
2573392
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
refractive index
n20/D 1.493 (lit.)
density
1.296 g/mL at 20 °C (lit.)
functional group
aldehyde
fluoro
SMILES string
[H]C(=O)c1cc(F)cc(F)c1
InChI
1S/C7H4F2O/c8-6-1-5(4-10)2-7(9)3-6/h1-4H
InChI key
ASOFZHSTJHGQDT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The 19F{′H} spectra of 3,5-difluorobenzaldehyde at low temperature in dimethyl ether solution was studied.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
132.8 °F - closed cup
flash_point_c
56 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A positive 6 J (H, CHO) in some meta derivatives of benzaldehyde. A simple model.
Canadian Journal of Chemistry, 67(5), 827-830 (1989)
Rasa Keruckiene et al.
Beilstein journal of organic chemistry, 16, 1142-1153 (2020-06-20)
Three compounds, bearing a quinazoline unit as the acceptor core and carbazole, dimethyldihydroacridine, or phenothiazine donor moieties, were designed and synthesized in two steps including a facile copper-catalyzed cyclization and a nucleophilic aromatic substitution reaction. The photophysical properties of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service