All Photos(2)
About This Item
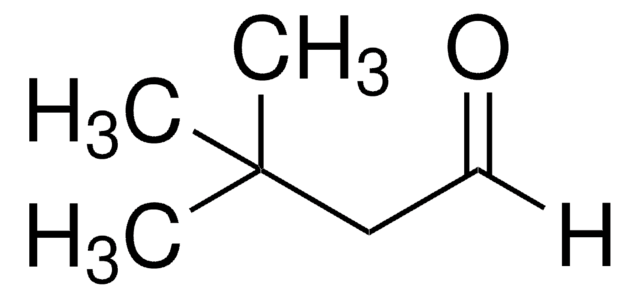
Linear Formula:
CH3CH(C6H5)CH2CHO
CAS Number:
Molecular Weight:
148.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.5179 (lit.)
bp
93-94 °C/16 mmHg (lit.)
density
0.997 g/mL at 25 °C (lit.)
functional group
aldehyde
phenyl
SMILES string
[H]C(=O)CC(C)c1ccccc1
InChI
1S/C10H12O/c1-9(7-8-11)10-5-3-2-4-6-10/h2-6,8-9H,7H2,1H3
InChI key
MYHGOWDLVRDUFA-UHFFFAOYSA-N
Application
3-Phenylbutyraldehyde was used as a reactant to study the kinetics of the reaction of hydrogen peroxide and certain aromatic aldehydes with cytochrome P450BM3-F87G.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
206.6 °F - closed cup
flash_point_c
97.00 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Holger Bühler et al.
Chembiochem : a European journal of chemical biology, 6(4), 711-717 (2005-04-07)
The influence of Trp128-substituted mutants of the hydroxynitrile lyase from Manihot esculenta (MeHNL) on the stereoselectivity of MeHNL-catalyzed HCN additions to aldehydes with stereogenic centers, which yield the corresponding cyanohydrins, is described. In rac-2-phenylpropionaldehyde (rac-1) reactions, wild-type (wtMeHNL) and all
G M Raner et al.
Journal of inorganic biochemistry, 81(3), 153-160 (2000-10-29)
The reaction of hydrogen peroxide and certain aromatic aldehydes with cytochrome P450BM3-F87G results in the covalent modification of the heme cofactor of this monooxygenase. Analysis of the resulting heme by electronic absorption spectrophotometry indicates that the reaction in the BM3
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service