280208
Dimethyl acetylsuccinate
Synonym(s):
2-Acetylbutanedioic acid-1,4-dimethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
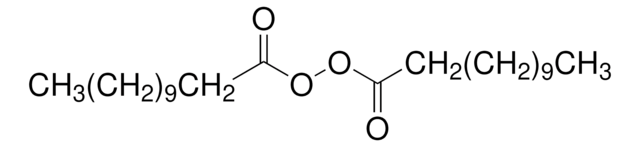
Linear Formula:
CH3O2CCH2CH(COCH3)CO2CH3
CAS Number:
Molecular Weight:
188.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
form
solid
bp
129-134 °C/12 mmHg (lit.)
mp
33 °C (lit.)
solubility
water: soluble 56.2 g/L at 20 °C
density
1.16 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
COC(=O)CC(C(C)=O)C(=O)OC
InChI
1S/C8H12O5/c1-5(9)6(8(11)13-3)4-7(10)12-2/h6H,4H2,1-3H3
InChI key
XREKLQOUFWBSFH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dimethyl acetylsuccinate undergoes sulfuric acid catalyzed Pechmann condensation with the corresponding resorcinol derivative to give 7-hydroxycoumarins.
Application
Dimethyl acetylsuccinate has been used in the synthesis of 2-oxofuro[2,3-b]pyrroles and 19-methyl-15-oxa-20-azatricyclo[12.3.3.0(1,14)]icos-18-en-18-carboxylates.
Reactant involved in:
- Cyclization for the synthesis of β-carbolines
- Synthesis of photochromic molecules with improved fatigue resistances and dark form stabilities
- Asymmetric hydrogenation of acylsuccinate followed by lactonization for synthesis of oxotetrahydrofurancarboxylates
- Amidation for synthesis of amino-acid derivatives of dihydropyranocoumarins
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_f
280.4 °F - closed cup
flash_point_c
138 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Raphael M Franzini et al.
Chembiochem : a European journal of chemical biology, 9(18), 2981-2988 (2008-11-28)
Templated nucleic acid detection is an emerging bioanalytical method that makes use of the target DNA or RNA strand to initiate a fluorogenic reaction. The Staudinger reduction holds particular promise for templated sensing of nucleic acids because the involved functional
Orazio A Attanasi et al.
The Journal of organic chemistry, 69(8), 2686-2692 (2004-04-13)
New and interesting 2-oxofuro[2,3-b]pyrroles and 19-methyl-15-oxa-20-azatricyclo[12.3.3.0(1,14)]icos-18-en-18-carboxylates have been obtained in good yields by the one-pot reaction, in basic medium, of 1,2-diaza-1,3-butadienes with diethyl or dimethyl acetylsuccinate or methyl 2-(1,3-dioxo-2-cyclotetradecyl)acetate, respectively, under mild conditions. Treatment of the same starting materials with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service