All Photos(1)
About This Item
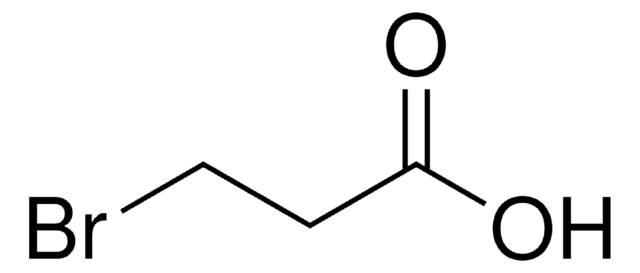
Linear Formula:
IC6H3(CH3)CO2H
CAS Number:
Molecular Weight:
262.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
210-212 °C (lit.)
SMILES string
Cc1ccc(cc1I)C(O)=O
InChI
1S/C8H7IO2/c1-5-2-3-6(8(10)11)4-7(5)9/h2-4H,1H3,(H,10,11)
InChI key
LDDHMKANNXWUAK-UHFFFAOYSA-N
Application
3-Iodo-4-methylbenzoic acid has been used in the preparation of:
- unlabeled N-succinimidyl 4-guanidinomethyl-3-iodobenzoate (SGIMB)
- boc-protected derivative of SGIMB (Boc-SGMIB)
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ganesan Vaidyanathan et al.
Nature protocols, 2(2), 282-286 (2007-04-05)
This protocol describes a detailed procedure for the synthesis of N-succinimidyl 4-guanidinomethyl-3-[*I]iodobenzoate ([*I]SGMIB), an agent useful in the radio-iodination of proteins, including monoclonal Abs, and peptides that undergo internalization after receptor or antigen binding. In this procedure, the tin precursor
G Vaidyanathan et al.
Bioconjugate chemistry, 12(3), 428-438 (2001-05-17)
The objective of this study was to develop an acylation agent for the radioiodination of monoclonal antibodies that would maximize retention of the label in tumor cells following receptor- or antigen-mediated internalization. The strategy taken was to add a polar
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service