276286
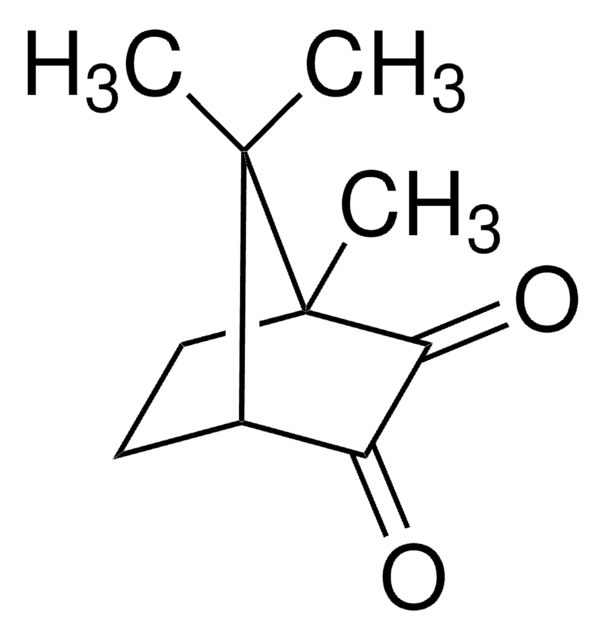
(1R)-(−)-Camphorquinone
99%
Synonym(s):
(1R)-(−)-2,3-Bornanedione, 2,3-Bornanedione
About This Item
Recommended Products
assay
99%
optical activity
[α]20/D −101°, c = 2 in toluene
mp
200-203 °C (lit.)
functional group
ketone
SMILES string
CC1(C)[C@@H]2CC[C@@]1(C)C(=O)C2=O
InChI
1S/C10H14O2/c1-9(2)6-4-5-10(9,3)8(12)7(6)11/h6H,4-5H2,1-3H3/t6-,10+/m1/s1
InChI key
VNQXSTWCDUXYEZ-LDWIPMOCSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- α-Hydroxycamphors by selective reduction of keto groups using various vegetables.
- Camphor-1,2-diamine platinum(II) complexes for DNA interaction studies.
- Camphoric anhydride by unsensitized photo-oxidation in the presence of oxygen and polar solvents.
- Camphorquinone-based chiral homoallylic amine, which is reacted with aldehydes to produce homoallylic primary amines via imine formation followed by 2-azonia-Cope rearrangement.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service