All Photos(2)
About This Item
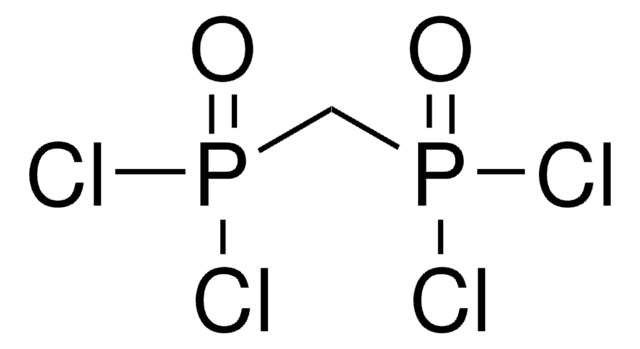
Linear Formula:
C2H5P(O)Cl2
CAS Number:
Molecular Weight:
146.94
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
98%
form
liquid
refractive index
n20/D 1.465 (lit.)
bp
71-72 °C/12 mmHg (lit.)
density
1.376 g/mL at 25 °C (lit.)
SMILES string
CCP(Cl)(Cl)=O
InChI
1S/C2H5Cl2OP/c1-2-6(3,4)5/h2H2,1H3
InChI key
OWGJXSYVHQEVHS-UHFFFAOYSA-N
Application
Ethylphosphonic dichloride has been used in the preparation of:
- ethylphosphonic diisocyanate
- 4-acetylphenyl isopropyl ethylphosphonate
- 4-acetylphenyl phenyl ethylphosphonate
- 4-acetylphenyl cyclohexyl ethylphosphonate
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Oral - Skin Corr. 1B
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charity Nowlan et al.
Journal of the American Chemical Society, 128(49), 15892-15902 (2006-12-07)
An array of 16 enantiomeric pairs of chiral phosphate, phosphonate, and phosphinate esters was used to establish the breadth of the stereoselective discrimination inherent within the bacterial phosphotriesterase and 15 mutant enzymes. For each substrate, the leaving group was 4-hydroxyacetophenone
Organophosphorus Isocyanates.
Haven Jr AC.
Journal of the American Chemical Society, 78(4), 842-843 (1956)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service