All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H13ClO2
CAS Number:
Molecular Weight:
164.63
Beilstein/REAXYS Number:
1280441
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
≥97.0%
refractive index
n20/D 1.459
bp
80 °C/8 mmHg (lit.)
density
1.115 g/mL at 20 °C (lit.)
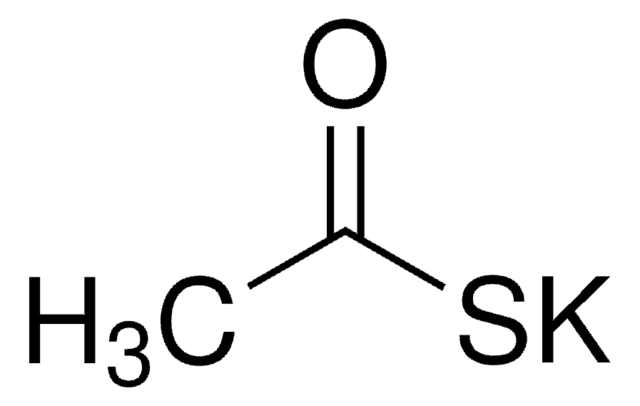
SMILES string
ClCCOC1CCCCO1
InChI
1S/C7H13ClO2/c8-4-6-10-7-3-1-2-5-9-7/h7H,1-6H2
InChI key
ZVVQFPGYDBUGQB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-(2-Chloroethoxy)tetrahydro-2H-pyran is a building block for introducing a protected 2-hydroxyethyl group.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
149.0 °F - closed cup
flash_point_c
65 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B. Son et al.
Synthesis, 776-776 (1984)
H.G. Lohr et al.
Chemische Berichte, 118, 914-914 (1985)
Michael Klein et al.
PloS one, 6(6), e20789-e20789 (2011-06-24)
Highly selective, cell-permeable and fast-acting inhibitors of individual kinases are sought-after as tools for studying the cellular function of kinases in real time. A combination of small molecule synthesis and protein mutagenesis, identified a highly potent inhibitor (1-Isopropyl-3-(phenylethynyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine) of a
H Ikuta et al.
Journal of medicinal chemistry, 30(11), 1995-1998 (1987-11-01)
A series of 3-(3,5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2-ones was synthesized and evaluated as candidate antiinflammatory/analgesic agents as well as dual inhibitors of prostaglandin and leukotriene synthesis. Some compounds that showed dual inhibitory activity were found to possess equipotent antiinflammatory activities to indomethacin, with reduced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service