All Photos(1)
About This Item
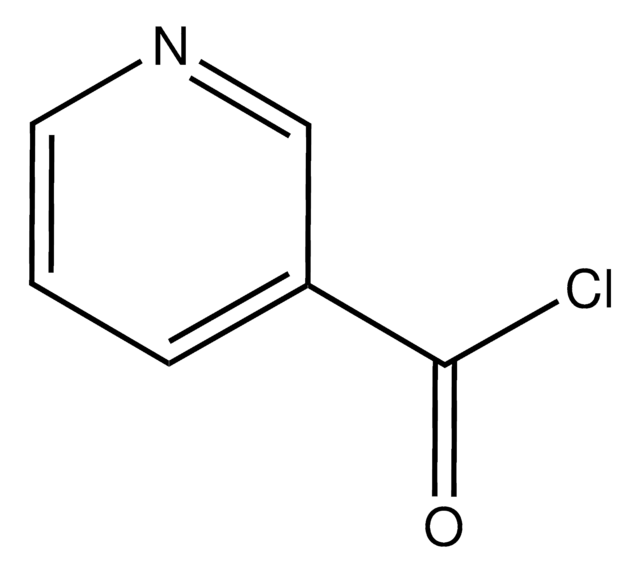
Empirical Formula (Hill Notation):
C6H4ClNO · HCl
CAS Number:
Molecular Weight:
178.02
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
powder
mp
151-157 °C (lit.)
SMILES string
Cl[H].ClC(=O)c1cccnc1
InChI
1S/C6H4ClNO.ClH/c7-6(9)5-2-1-3-8-4-5;/h1-4H;1H
InChI key
MSYBLBLAMDYKKZ-UHFFFAOYSA-N
Application
Nicotinoyl chloride hydrochloride was used in the preparation of :
- 1-[5-O-tert-butyldimethylsilyl-2-deoxy-2-fluoro-3-O-(3-pyridylcarbonyl)-β-D-ribofuranosyl]uracil
- 7,16-dinicotinoylated and/or 7,16-diisonicotinoylated compounds via reaction with tetraaza[14]annulene and its complexes
- bis(ethylenedithio)tetrathiafulvalene derivatives
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K W Morin et al.
Carbohydrate research, 249(1), 109-116 (1993-10-18)
1-(2-Deoxy-2-fluoro-beta-D-ribofuranosyl-(E)-5-(2-iodovinyl)uracil (IVFRU) was coupled to a dihydropyridine <---> pyridinium salt redox chemical-delivery system (CDS) via a cleavable sugar-ester linkage as a site-directed approach to increase diffusion of the parent nucleoside into the central nervous system. Treatment of 1-(2-deoxy-2-fluoro-beta-D-ribofuranosyl)uracil with Bu(t)Me(2)SiCl
Synthesis of bis(ethylenedithio) tetrathiafulvalene derivatives with metal ion ligating centres.
Griffiths J-P, et al.
Tetrahedron Letters, 44(15), 31327-31331 (2003)
Shuang Li et al.
Journal of the mechanical behavior of biomedical materials, 102, 103521-103521 (2019-12-27)
In this research, a bio-based monomer 1,3-bis(methacryloyloxy)propyl-carbonyl- hexylpyridinium bromide (QANMA) that derived from niacin was synthesized and incorporated into Bisphenol A glycidyl methacrylate (Bis-GMA)/triethylene glycol dimethacrylate (TEGDMA) (50 wt/50 wt) with a series of mass fraction as antibacterial agent. The double bond
Jingjing Zhang et al.
Marine drugs, 18(3) (2020-03-20)
Chitosan is an active biopolymer, and the combination of it with other active groups can be a valuable method to improve the potential application of the resultant derivatives in food, cosmetics, packaging materials, and other industries. In this paper, a
Wenqiang Tan et al.
Carbohydrate polymers, 157, 236-243 (2016-12-19)
Based on cuprous-catalyzed azide-alkyne cycloaddition (CuAAC), starch derivative bearing 1,2,3-triazole and pyridine (II) was prepared and subsequently followed by alkylation with iodomethane to synthesize starch derivative bearing 1,2,3-triazolium and pyridinium (III). The antifungal activities of starch derivatives against Colletotrichum lagenarium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service