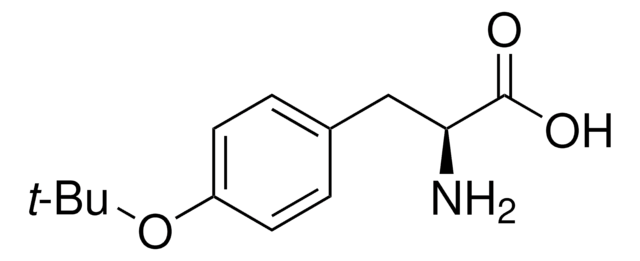
20589
O-tert-Butyl-L-serine tert-butyl ester hydrochloride
≥98.0% (AT)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H23NO3 · HCl
CAS Number:
Molecular Weight:
253.77
Beilstein/REAXYS Number:
4886392
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98.0% (AT)
form
solid
optical activity
[α]/D -10.0±1.0°, c = 1 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
SMILES string
Cl.CC(C)(C)OC[C@H](N)C(=O)OC(C)(C)C
InChI
1S/C11H23NO3.ClH/c1-10(2,3)14-7-8(12)9(13)15-11(4,5)6;/h8H,7,12H2,1-6H3;1H/t8-;/m0./s1
InChI key
RDWZQVGVBTYCBD-QRPNPIFTSA-N
Other Notes
Protected L-serine used in peptide synthesis, e.g. synthesis of glycopeptides and lipopeptides; Preparation of amino-phospholipids
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H. Paulsen et al.
Liebigs Ann. Chem., 751-751 (1989)
Y Tsuda et al.
Chemical & pharmaceutical bulletin, 39(3), 607-611 (1991-03-01)
The structure of WS1279, isolated from Streptomyces sp. as an immunoactive lipopeptide, has been deduced on the basis of chemical and physical evidence as S-[2,3-bis(palmitoyloxy)propyl]-N alpha-palmitoyl-Cys-Asn-Ser-Gly-Gly-Ser- OH. This was confirmed by synthesis.
Liebigs Ann. Chem., 771-771 (1989)
J G Turcotte et al.
Chemistry and physics of lipids, 58(1-2), 81-95 (1991-05-01)
A homologous series of chiral (R) ether-amide phosphonolipid analogs of naturally occurring (R) glycerophospholipids were synthesized and characterized for their interfacial behaviors. The phosphonolipids possess isoteric ether, amide, and phosphonate functions at positions corresponding to the sn-1, sn-2, and sn-3
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service