190438
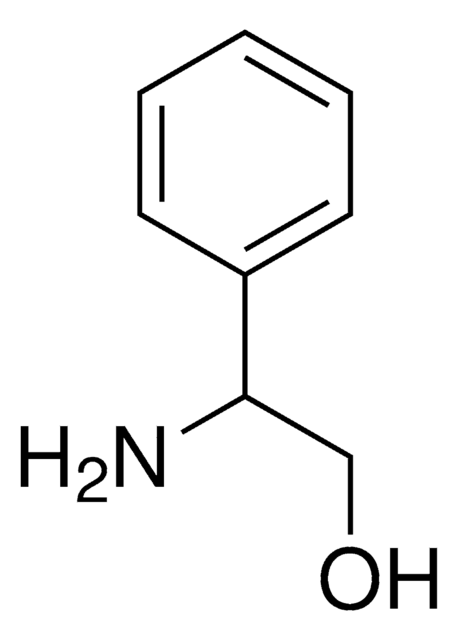
(S)-(−)-2-Amino-3-phenyl-1-propanol
98%, optical purity ee: 99% (HPLC)
Synonym(s):
L-Phenylalaninol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2CH(NH2)CH2OH
CAS Number:
Molecular Weight:
151.21
Beilstein/REAXYS Number:
2208238
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
solid
optical activity
[α]22/D −22.8°, c = 1.2 in 1 M HCl
optical purity
ee: 99% (HPLC)
mp
92-94 °C (lit.)
functional group
amine
hydroxyl
phenyl
SMILES string
N[C@H](CO)Cc1ccccc1
InChI
1S/C9H13NO/c10-9(7-11)6-8-4-2-1-3-5-8/h1-5,9,11H,6-7,10H2/t9-/m0/s1
InChI key
STVVMTBJNDTZBF-VIFPVBQESA-N
Related Categories
General description
(S)-(-)-2-Amino-3-phenyl-1-propanol is a chiral amino alcohol.
Application
(S)-(-)-2-Amino-3-phenyl-1-propanol can undergo condensation with 5-nitrosalicylaldehyde to form (S)-2-[(1-benzyl-2-hydroxyethylimino)methyl]-4-nitrophenol, a new chiral Schiff base. It reacts with substituted salicylaldehydes to form tridentate chiral Schiff base ligands, which can form H-bonded chiral supramolecular metal-organic architectures. It can also be used in the synthesis of an unnatural tripeptide, which can enhance the antimicrobial activity of methicillin against methicillin resistant Staphylococcus aureus.
Reacts with nitriles to form oxazolines which are useful in Pd-catalyzed allylic substitution. Also employed in amidation for chiral resolution and NADH modeling.
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Intramolecular hydrogen transfer in (S)-2-[(1-benzyl-2-hydroxyethylimino) methyl]-4-nitrophenol, a new chiral Schiff base.
Pradeep CP.
Acta Crystallographica Section E, Structure Reports Online, 61(11), o3825-o3827 (2005)
Tetrahedron Letters, 34, 7081-7081 (1993)
Chiral supramolecular metal-organic architectures from dinuclear copper complexes.
Pradeep CP and Das SK.
Polyhedron, 28(3), 630-636 (2009)
Tetrahedron Letters, 34, 2015-2015 (1993)
W W Mak et al.
Canadian journal of biochemistry, 58(9), 737-744 (1980-09-01)
This study describes a rounding reaction induced in mammalian cells by the addition of phenylalaninol. In the Chinese hamster ovary tsH1 line the rounding occurred rapidly with a half time of 1 min at 25 mM phenylalaninol. After the removal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service