15299
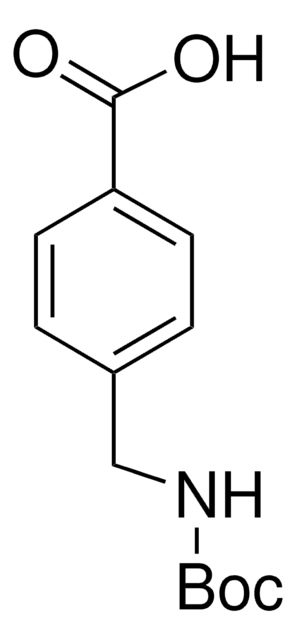
Boc-4-Abz-OH
≥98.0% (T)
Synonym(s):
4-(Boc-amino)benzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3CO2CNHC6H4CO2H
CAS Number:
Molecular Weight:
237.25
Beilstein/REAXYS Number:
2115614
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
Recommended Products
assay
≥98.0% (T)
reaction suitability
reaction type: Boc solid-phase peptide synthesis
mp
~200 °C (dec.)
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)Nc1ccc(cc1)C(O)=O
InChI
1S/C12H15NO4/c1-12(2,3)17-11(16)13-9-6-4-8(5-7-9)10(14)15/h4-7H,1-3H3,(H,13,16)(H,14,15)
InChI key
ZJDBQMWMDZEONW-UHFFFAOYSA-N
Other Notes
BOC-protected derivative of PABA.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M H Norman et al.
Journal of medicinal chemistry, 39(5), 1172-1188 (1996-03-01)
A series of substituted (4-(4-(1,2-benzisothiazol-3-yl)-1-piperazinyl)butyl)benzamide derivatives was prepared and evaluated as potential atypical antipsychotic agents. The target compounds were readily prepared from their benzoyl chloride, benzoic acid, or isatoic anhydride precursors, and they were evaluated in vitro for their ability
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service