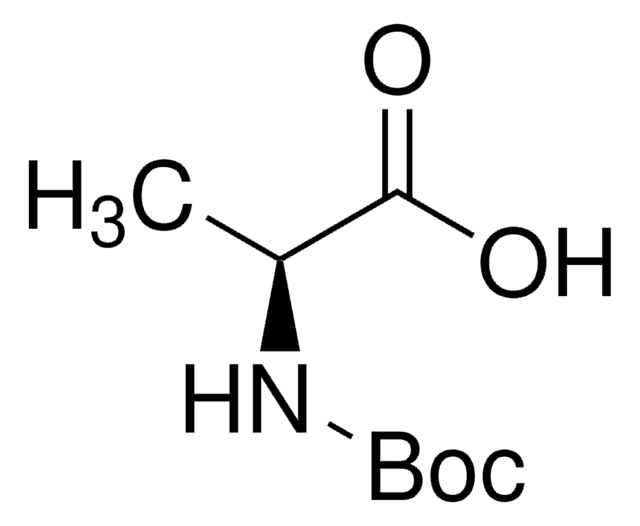
15185
Boc-D-Trp-OH
≥98.0% (TLC)
Synonym(s):
Nα-Boc-D-tryptophan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H20N2O4
CAS Number:
Molecular Weight:
304.34
Beilstein/REAXYS Number:
4237334
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98.0% (TLC)
reaction suitability
reaction type: Boc solid-phase peptide synthesis
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI
1S/C16H20N2O4/c1-16(2,3)22-15(21)18-13(14(19)20)8-10-9-17-12-7-5-4-6-11(10)12/h4-7,9,13,17H,8H2,1-3H3,(H,18,21)(H,19,20)/t13-/m1/s1
InChI key
NFVNYBJCJGKVQK-CYBMUJFWSA-N
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y Kato et al.
Biochemical and biophysical research communications, 234(1), 82-84 (1997-05-08)
The aim of this study was to clarify the mechanism of loss of Trp residues in proteins exposed to peroxynitrite. The Trp residues in bovine serum albumin and collagen IV were decreased by peroxynitrite treatment. To identify the degradation products
R Vespalec et al.
Electrophoresis, 19(2), 276-281 (1998-04-21)
An equation for the calculation of electrophoretic mobility of kinetically labile complexes originating in solutions during the chiral discrimination process is derived. The mobility of the complex is calculated from that of a fully ionized racemic compound, measured in absence
R Magous et al.
Biochimica et biophysica acta, 845(2), 158-162 (1985-05-30)
Benzotript (N-p-chlorobenzoyl-L-tryptophan) has been shown to be a receptor-antagonist in vivo and in vitro for peptides from the gastrin family. In the present study, we examine tryptophan, and some of its N- and C-acylated derivatives, as well as some phenylalanine
Denisa Folprechtová et al.
Electrophoresis, 40(15), 1972-1977 (2019-01-24)
Three chiral stationary phases were prepared by dynamic coating of sulfobutylether-β-cyclodextrin (SBE-β-CD) with different degrees of substitution, onto strong anion-exchange stationary phases. The enantioselective potential and stability of newly prepared chiral stationary phases were examined using a set of structurally
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service