All Photos(1)
About This Item
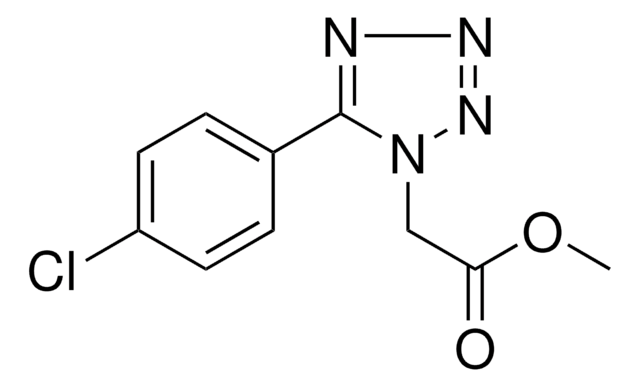
Empirical Formula (Hill Notation):
C7H5ClN4
CAS Number:
Molecular Weight:
180.59
Beilstein/REAXYS Number:
139070
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
120-123 °C (lit.)
solubility
dichloromethane: soluble 25 mg/mL, clear, colorless
SMILES string
Clc1nnnn1-c2ccccc2
InChI
1S/C7H5ClN4/c8-7-9-10-11-12(7)6-4-2-1-3-5-6/h1-5H
InChI key
DHELIGKVOGTMGF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Chloro-1-phenyl-1H-tetrazole reacts with 2-hydroxyacetophenone to yield 1-phenyl-1H-tetrazol-5-oxy linked compound.
Application
5-Chloro-1-phenyl-1H-tetrazole was used as the reagent during the synthesis of new glycosyl donor possessing an anomeric O-(1-phenyltetrazol-5-yl) group.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Palme et al.
Bioorganic & medicinal chemistry, 2(11), 1169-1177 (1994-11-01)
A new glycosyl donor possessing an anomeric O-(1-phenyltetrazol-5-yl) group is prepared from 2,3,4,6-tetra-O-benzyl-D-glucose (2) and commercially available 5-chloro-1-phenyl-1H-tetrazole (1). The synthesis of glycosides derived from the donor and a few primary and secondary alcohols is reported.
Scott P Webster et al.
Bioorganic & medicinal chemistry letters, 20(11), 3265-3271 (2010-05-11)
Inhibitors of 11beta-hydroxysteroid dehydrogenase (11beta-HSD1) show promise as drugs to treat metabolic disease and CNS disorders such as cognitive impairment. A series of 1,5-substituted 1H-tetrazole 11beta-HSD1 inhibitors has been discovered and chemically modified. Compounds are selective for 11beta-HSD1 over 11beta-HSD2
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service