All Photos(1)
About This Item
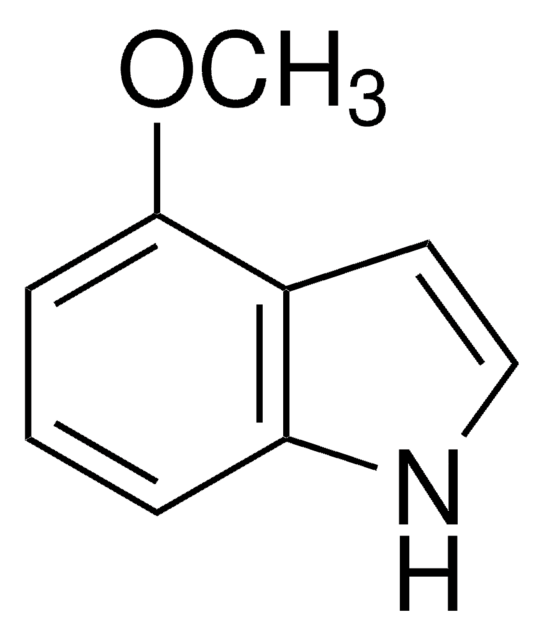
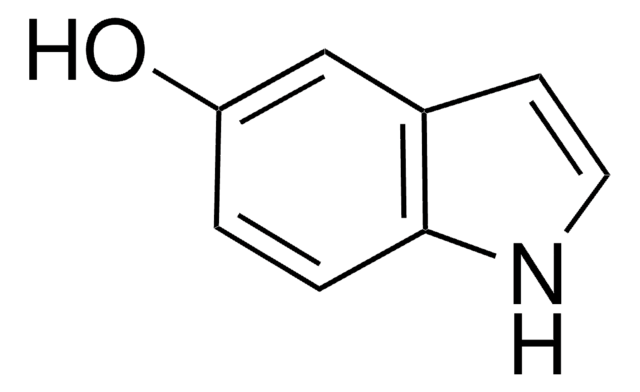
Empirical Formula (Hill Notation):
C9H9NO
CAS Number:
Molecular Weight:
147.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
assay
98%
mp
90-92 °C (lit.)
SMILES string
COc1ccc2cc[nH]c2c1
InChI
1S/C9H9NO/c1-11-8-3-2-7-4-5-10-9(7)6-8/h2-6,10H,1H3
InChI key
QJRWYBIKLXNYLF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Methoxyindole was used to study the fluorescence of protonated excited-state forms of serotonin and other related compounds in acid. It was used in the preparation of:
- tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles, potential anticancer immunomodulator
- indolylindazoles and indolylpyrazolopyridines, interleukin-2 inducible T cell kinase inhibitors
- diindolyloxyindoles, anticancer agents
- benzoylpiperazinyl-indolyl ethane dione derivatives, HIV-1 inhibitors
- 3-aroylindoles as anticancer agents
- indolyl and isoquinolinyl anthranilates, PPARδ partial agonists
- heteroaryl ketones, VEGFR-2 inhibitors
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for synthesis of indolylindazoles and indolylpyrazolopyridines as interleukin-2 inducible T cell kinase inhibitors
- Reactant for preparation of diindolyloxyindoles as anticancer agents
- Reactant for preparation of benzoylpiperazinyl-indolyl ethane dione derivatives as HIV-1 inhibitors
- Reactant for preparation of 3-aroylindoles as anticancer agents
- Reactant for preparation of indolyl and isoquinolinyl anthranilates as PPARδ partial agonists
- Reactant for preparation of heteroaryl ketones as VEGFR-2 inhibitors
Biochem/physiol Actions
6-Methoxyindole inhibits the chlorinating activity of myeloperoxidase and inhibits the generation of hypochlorous acid released by activated leukocytes.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eugene L Piatnitski Chekler et al.
Bioorganic & medicinal chemistry letters, 18(15), 4344-4347 (2008-07-22)
We have discovered novel inhibitors of VEGFR-2 kinase with low nanomolar potency in both enzymatic and cell-based assays. Active series are heteroaryl-ketone compounds containing a central aromatic ring with either an indazolyl or indolyl keto group in the ortho orientation
Ahmed Kamal et al.
Bioorganic & medicinal chemistry letters, 20(17), 5229-5231 (2010-08-03)
A simple and highly efficient method has been developed for the synthesis of 3,3-diindolyl oxyindoles by the reaction of indoles with isatin or 5-fluoro isatin using a catalytic amount (5 mol%) of FeCl(3) at room temperature in a short reaction
V F Ximenes et al.
Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 38(11), 1575-1583 (2005-11-01)
Hypochlorous acid (HOCl) released by activated leukocytes has been implicated in the tissue damage that characterizes chronic inflammatory diseases. In this investigation, 14 indole derivatives, including metabolites such as melatonin, tryptophan and indole-3-acetic acid, were screened for their ability to
Fluorescence of protonated excited-state forms of 5-hydroxytryptamine (serotonin) and related indoles.
R F Chen
Proceedings of the National Academy of Sciences of the United States of America, 60(2), 598-605 (1968-06-01)
Barry G Shearer et al.
Bioorganic & medicinal chemistry letters, 18(18), 5018-5022 (2008-08-30)
Anthranilic acid GW9371 was identified as a novel class of PPARdelta partial agonist through high-throughput screening. The design and synthesis of SAR analogues is described. GSK1115 and GSK7227 show potent partial agonism of the PPARdelta target genes CPT1a and PDK4
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service