116289
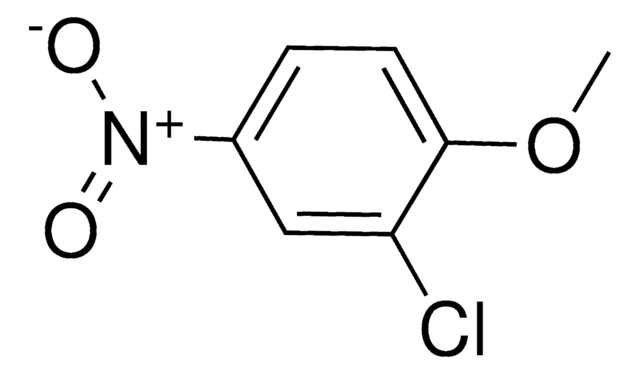
4-Chloro-3-nitroanisole
98%
Synonym(s):
1-Chloro-4-methoxy-2-nitrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H3(NO2)OCH3
CAS Number:
Molecular Weight:
187.58
Beilstein/REAXYS Number:
640872
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
41-43 °C (lit.)
functional group
chloro
nitro
SMILES string
COc1ccc(Cl)c(c1)[N+]([O-])=O
InChI
1S/C7H6ClNO3/c1-12-5-2-3-6(8)7(4-5)9(10)11/h2-4H,1H3
InChI key
HISHUMDTGXICEZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-chloro-3-nitroanisole is used to synthesize a group of derivatives of 7-methanesulfonylamino-6-phenoxychromone at the pyrone and phenoxy rings.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Inaba et al.
Chemical & pharmaceutical bulletin, 48(1), 131-139 (2000-03-08)
A group of derivatives of 7-methanesulfonylamino-6-phenoxychromone (1) at the pyrone and phenoxy rings was synthesized starting with 4-chloro-3-nitroanisole and evaluated against acute and chronic inflammations in oral administration in animals. Significant potency in the rat models of carrageenin-induced edema (CPE)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service